
Gerald's science teacher mixed room temperature samples of hydrochloric acid and sodium hydroxide in a large beaker. the solution still looked clear like water, but when the students carefully touched the beaker one at a time, it felt warm to the touch. why did the beaker most likely feel warm? a. the two liquids were not soluble in water. b. the release of a gas heated the solution. c. the energy of mixing warmed the solution. d. a chemical reaction was producing a new substance.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
You know the right answer?
Gerald's science teacher mixed room temperature samples of hydrochloric acid and sodium hydroxide in...
Questions

Chemistry, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01


Biology, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01

Computers and Technology, 20.09.2020 05:01



History, 20.09.2020 05:01

Biology, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01


Biology, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01

English, 20.09.2020 05:01


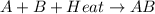
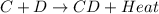 is an exothermic reaction.
is an exothermic reaction.

