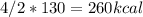Chemistry, 27.06.2019 15:30 hahalol123goaway
Calculate the enthalpy change when 4.00 mol cl2o7 is produced according to the following balanced equation: 2cl2(g) + 7o2(g) + 130kcal -> 2cl2o7(g) a. 1040 kcal b. -260 kcal c. 260 kcal d. -1040 kcal ** if you could explain it as well, that would be much appreciated if not, thats okay too its multiple choice

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
Calculate the enthalpy change when 4.00 mol cl2o7 is produced according to the following balanced eq...
Questions

Biology, 16.09.2019 17:10

History, 16.09.2019 17:10

Social Studies, 16.09.2019 17:10

Social Studies, 16.09.2019 17:10

Physics, 16.09.2019 17:10



Biology, 16.09.2019 17:10



Physics, 16.09.2019 17:10









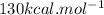
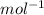 is mentioned because it is for per mole of reaction. So for 4. moles of the product
is mentioned because it is for per mole of reaction. So for 4. moles of the product 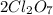 we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.
we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.