
Chemistry, 27.06.2019 19:30 carriganlee8688
The equation below shows the decomposition of lead nitrate. how many grams of oxygen are produced when 11.5g no2 is formed? 2pb(no3)2(s) -> 2pbo(s) + 4no2(g) + o2(g)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
The equation below shows the decomposition of lead nitrate. how many grams of oxygen are produced wh...
Questions

Business, 04.02.2021 22:20

History, 04.02.2021 22:20


Mathematics, 04.02.2021 22:20

Social Studies, 04.02.2021 22:20

Mathematics, 04.02.2021 22:20


History, 04.02.2021 22:20

Mathematics, 04.02.2021 22:20

Law, 04.02.2021 22:20


Mathematics, 04.02.2021 22:20

Biology, 04.02.2021 22:20

Mathematics, 04.02.2021 22:20






Mathematics, 04.02.2021 22:20

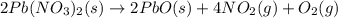
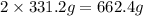 of lead nitrate produces
of lead nitrate produces 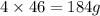 of nitrogen dioxide.
of nitrogen dioxide.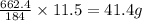 of lead nitrate
of lead nitrate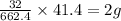 of oxygen.
of oxygen.

