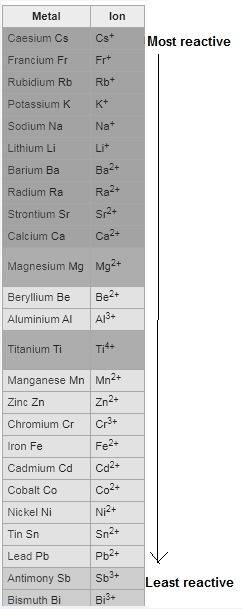
Question 1 (true/false worth 2 points) (04.03 lc) when pb and alcl3 react together, lead (pb) can replace aluminum (al) in the compound because lead is lower on the activity series. true false question 2(multiple choice worth 4 points) (04.03 mc) which of the following equations has the correct products and is balanced correctly for a reaction between na3po4 and koh? na3po4 + 3koh → 3naoh + k3po4, because k retains the same charge throughout the reaction na3po4 + koh → na3oh + kpo4, because k increases in charge from 1+ to 3+ when it is replaced na3po4 + koh → 3naoh + k3po4, because k retains the same charge throughout the reaction na3po4 + koh → na3oh + k3po4, because k increases in charge from 1+ to 3+ when it is replaced question 3(multiple choice worth 4 points) (04.03 lc) which of the following is a single replacement reaction? ba(oh)2 + h2so4 → baso4 + 2h2o 2mg + o2 → 2mgo h2o+ co2 → h2co3 zn + h2so4 → znso4 + h2 question 4(multiple choice worth 4 points) (04.03 mc) sodium metal reacts with water to produce hydrogen gas. what best describes this reaction? a single replacement reaction takes place because sodium is less reactive than hydroxide ions. a double replacement reaction takes place because sodium is less reactive than hydroxide ions. a double replacement reaction takes place because sodium is more reactive than hydrogen. a single replacement reaction takes place because sodium is more reactive than hydrogen. question 5 (true/false worth 2 points) (04.03 lc) a single replacement reaction is a reaction in which one element replaces a similar element within a compound. true false question 6(multiple choice worth 4 points) (04.03 mc) the table shows the nature of reactants and products formed in a certain type of chemical reaction. nature of reactants and products reactants products metal + ionic compound metal + ionic compound which of the following is true about the type of chemical reaction? it is a single replacement reaction, and the anions in the two ionic compounds are different. it is a single replacement reaction, and the cations in the two ionic compounds are different. it is a double replacement reaction, and the anions in the two ionic compounds are different. it is a double replacement reaction, and the cations in the two ionic compounds are different.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3


Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
Question 1 (true/false worth 2 points) (04.03 lc) when pb and alcl3 react together, lead (pb) can re...
Questions


Business, 17.06.2020 20:57







Mathematics, 17.06.2020 20:57



Mathematics, 17.06.2020 20:57





Mathematics, 17.06.2020 20:57


Mathematics, 17.06.2020 20:57

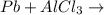 no reaction
no reaction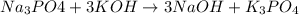 , because K retains the same charge throughout the reaction.
, because K retains the same charge throughout the reaction.  as well as
as well as 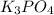
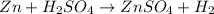
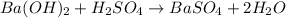 : is a double displacement reaction in which ion exchange takes place.
: is a double displacement reaction in which ion exchange takes place. 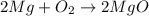 : It is a synthesis reaction as two reactants combine to give a single product.
: It is a synthesis reaction as two reactants combine to give a single product.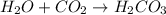 : It is a synthesis reaction as two reactants combine to give a single product.
: It is a synthesis reaction as two reactants combine to give a single product.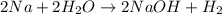 Sodium metal reacts with water to produce hydrogen gas: A single replacement reaction takes place because sodium is more reactive than hydrogen.
Sodium metal reacts with water to produce hydrogen gas: A single replacement reaction takes place because sodium is more reactive than hydrogen. 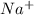 in NaOH and
in NaOH and 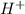 get reduced to give
get reduced to give 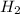
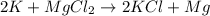 where K is a metal and
where K is a metal and 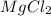 is an ionic compound. K being more reactive than Mg, displaces it from its salt solution.
is an ionic compound. K being more reactive than Mg, displaces it from its salt solution.