Chemistry, 27.06.2019 22:30 yehnerthannah
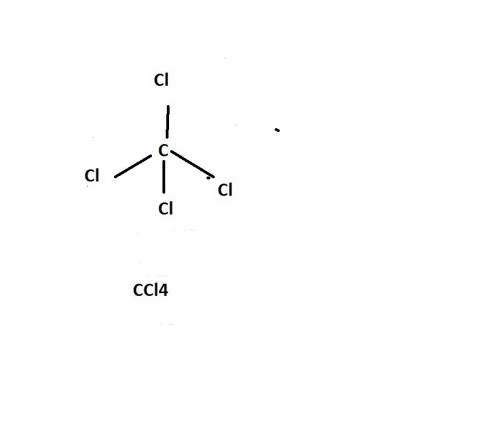
Chem carbon tetrachloride has been widely used in the cleaning industry, in fire extinguishers, and as a refrigerant. construct an explanation of how carbon and chlorine combine to form carbon tetrachloride. a) nonmetal carbon shares valence electrons with each nonmetal chlorine forming four covalent bonds. b) nonmetal carbon loses a valence electron and chlorine metal gains a valence electron to form an ionic bond. c) carbon and chlorine are nonmetals and they shares their valence electrons to become ions and form ionic bonds. d) chlorine metal loses a valence electron to become a cation and nonmetal carbon gains a valence electron to become an anion forming a covalent bond.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
Chem carbon tetrachloride has been widely used in the cleaning industry, in fire extinguishers, and...
Questions

Social Studies, 02.01.2022 02:20

Mathematics, 02.01.2022 02:20

Mathematics, 02.01.2022 02:20


History, 02.01.2022 02:20

Advanced Placement (AP), 02.01.2022 02:20



History, 02.01.2022 02:20

Mathematics, 02.01.2022 02:20

Mathematics, 02.01.2022 02:20

World Languages, 02.01.2022 02:20


Computers and Technology, 02.01.2022 02:20

Physics, 02.01.2022 02:20


Mathematics, 02.01.2022 02:30