
Chemistry, 28.06.2019 03:00 jetblackcap
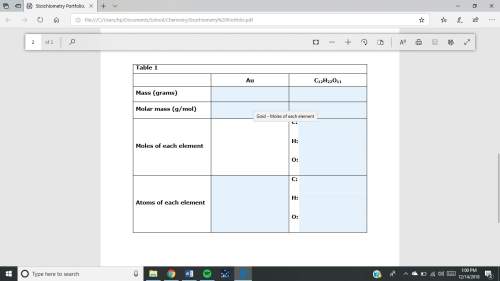
1. a sample of gold (au) has a mass of 35.12 g. a. calculate the number of moles of gold (au) in the sample and record in table 1. show your work. b. calculate the number of atoms of gold (au) in the sample and record in table 1. show your work. 2. a sample of table sugar (sucrose, c12h22o11) has a mass of 1.202 g. a. calculate the number of moles of c12h22o11 contained in the sample and record in table 1. show your work. b. calculate the moles of each element in c12h22o11 and record in table 1. show your work. c. calculate the number of atoms of each type in c12h22o11 and record in table 1. show your work. table looks like this: column 1 column 2 column 3 au c12h22o11mass (grams)molar mass (g/mol)moles of each element c: h: o: atoms of each element c: h: o: fast!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2



You know the right answer?
1. a sample of gold (au) has a mass of 35.12 g. a. calculate the number of moles of gold (au) in the...
Questions





Mathematics, 27.07.2019 00:30



History, 27.07.2019 00:30


English, 27.07.2019 00:30


Mathematics, 27.07.2019 00:30





Mathematics, 27.07.2019 00:30

English, 27.07.2019 00:30

English, 27.07.2019 00:30



