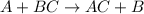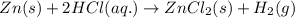Zinc metal reacts with hydrochloric acid (hcl) to produce hydrogen gas. what best describes this reaction? a single replacement reaction takes place because zinc is more reactive than hydrogen. a single replacement reaction takes place because zinc is more reactive than chlorine. a double replacement reaction takes place because zinc is less reactive than hydrogen. a double replacement reaction takes place because zinc is less reactive than chlorine.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
Zinc metal reacts with hydrochloric acid (hcl) to produce hydrogen gas. what best describes this rea...
Questions

History, 22.10.2020 04:01

English, 22.10.2020 04:01

Law, 22.10.2020 04:01

Business, 22.10.2020 04:01


Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01




Computers and Technology, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01

Social Studies, 22.10.2020 04:01


Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01