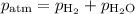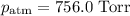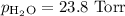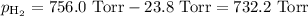Chemistry, 28.06.2019 07:30 emmaty7845
A0.630 gram sample of a metal, m, reacts completely with sulfuric acid according to: a volume of 291 ml of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. atmospheric pressure is 756.0 torr and the temperature is 25 °c. the vapor pressure of water at various temperatures can be found in this table. calculate the molar mass of the metal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2


Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
A0.630 gram sample of a metal, m, reacts completely with sulfuric acid according to: a volume of 291...
Questions

Biology, 27.05.2020 04:00


Mathematics, 27.05.2020 04:00

Biology, 27.05.2020 04:00

Mathematics, 27.05.2020 04:00

Mathematics, 27.05.2020 04:00

Mathematics, 27.05.2020 04:00

History, 27.05.2020 04:00

Mathematics, 27.05.2020 04:00

History, 27.05.2020 04:00


Mathematics, 27.05.2020 04:00

English, 27.05.2020 04:00

English, 27.05.2020 04:00

Mathematics, 27.05.2020 04:00