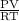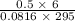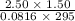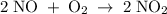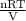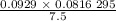Chemistry, 28.06.2019 07:30 nickeymcorrea
The picture below shows two bulbs connected by a stopcock. the large bulb, with a volume of 6.00 l, contains nitric oxide at a pressure of 0.500 atm, and the small bulb, with a volume of 1.50 l, contains oxygen at a pressure of 2.50 atm. the temperature at the beginning and the end of the experiment is 22 °c. what are the partial gasses of no, and no2?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
The picture below shows two bulbs connected by a stopcock. the large bulb, with a volume of 6.00 l,...
Questions







English, 27.10.2020 01:00


Mathematics, 27.10.2020 01:00

History, 27.10.2020 01:00





History, 27.10.2020 01:00


Mathematics, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00

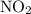 is 0.400 atm at the end of the reaction.
is 0.400 atm at the end of the reaction.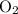 initially are;
initially are;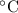 = 273 + 22 K = 295 K
= 273 + 22 K = 295 K