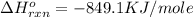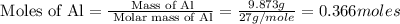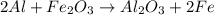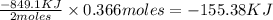Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1


Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
Determine the heat given off to the surroundings when 9.873 g of aluminum reacts according to the eq...
Questions

Mathematics, 01.09.2020 19:01

Mathematics, 01.09.2020 19:01

Biology, 01.09.2020 19:01


Mathematics, 01.09.2020 19:01

Mathematics, 01.09.2020 19:01



Biology, 01.09.2020 19:01

Business, 01.09.2020 19:01

Mathematics, 01.09.2020 19:01

Biology, 01.09.2020 19:01

Physics, 01.09.2020 19:01


Mathematics, 01.09.2020 19:01

Social Studies, 01.09.2020 19:01

Mathematics, 01.09.2020 19:01

Mathematics, 01.09.2020 19:01

History, 01.09.2020 19:01

Mathematics, 01.09.2020 19:01