Chemistry, 28.06.2019 17:30 iilovejohnccena1022
When 25.0 grams of water are cooled from 20.0 degrees celsius to 10.0 degrees celsius the number of joules of heat energy released is?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
The electron configurations of two different atoms are shown below. each yellow electron has a charge of 1−, and the net charge of each nucleus is shown. these atoms will combine with bond. a. an ionic b. a positive c. a negative d. a covalent plzzz mee with !
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3
You know the right answer?
When 25.0 grams of water are cooled from 20.0 degrees celsius to 10.0 degrees celsius the number of...
Questions

Health, 27.02.2021 01:10


Mathematics, 27.02.2021 01:10



Chemistry, 27.02.2021 01:10

World Languages, 27.02.2021 01:10






Mathematics, 27.02.2021 01:10

Business, 27.02.2021 01:10

Mathematics, 27.02.2021 01:10


English, 27.02.2021 01:10

Mathematics, 27.02.2021 01:10


Mathematics, 27.02.2021 01:10

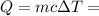
 = Change in temperature
= Change in temperature