
Chemistry, 28.06.2019 17:30 ehsaangaminglegend
Agalvanic cell has the overall reaction: zn(s) 2eu(no3)3(aq) zn(no3)2(aq) 2eu(no3)2(aq) which is the half reaction occurring at the anode

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

You know the right answer?
Agalvanic cell has the overall reaction: zn(s) 2eu(no3)3(aq) zn(no3)2(aq) 2eu(no3)2(aq) which is...
Questions


Social Studies, 06.05.2020 04:24



Mathematics, 06.05.2020 04:24

English, 06.05.2020 04:24

History, 06.05.2020 04:24

Health, 06.05.2020 04:24


Mathematics, 06.05.2020 04:24

Mathematics, 06.05.2020 04:24






Social Studies, 06.05.2020 04:24



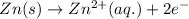
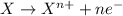
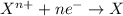
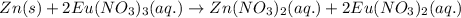
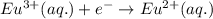 ( × 2 )
( × 2 )

