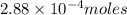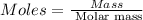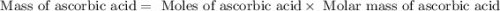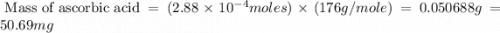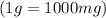Chemistry, 28.06.2019 17:30 kprincess16r
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compound with antioxidant properties. a healthy adult’s daily requirement of vitamin c is 70-90 mg. a sweet lime contains 2.88×10−4 mol of ascorbic acid. to determine whether the ascorbic acid in a sweet lime meets the daily requirement, calculate the mass of ascorbic acid in 2.88×10−4 mol of ascorbic acid.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2


Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compo...
Questions

History, 01.08.2019 17:30


History, 01.08.2019 17:30

History, 01.08.2019 17:30


Mathematics, 01.08.2019 17:30





History, 01.08.2019 17:30


English, 01.08.2019 17:30

Mathematics, 01.08.2019 17:30


Mathematics, 01.08.2019 17:30

Mathematics, 01.08.2019 17:30



Mathematics, 01.08.2019 17:30