
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of solid and liquid ethanol are 0.97 j/gk and 2.3 j/gk, respectively. how much heat (kj) is needed to convert 25.0 g of solid ethanol at -135â°c to liquid ethanol at -50â°c?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of soli...
Questions

Mathematics, 29.01.2021 20:10

Mathematics, 29.01.2021 20:10

Mathematics, 29.01.2021 20:10



History, 29.01.2021 20:10

Spanish, 29.01.2021 20:10

History, 29.01.2021 20:10

Mathematics, 29.01.2021 20:10

Chemistry, 29.01.2021 20:10

Advanced Placement (AP), 29.01.2021 20:10




Chemistry, 29.01.2021 20:10

Mathematics, 29.01.2021 20:10




History, 29.01.2021 20:10

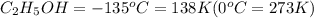
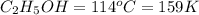
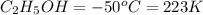
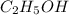 at 159 K
at 159 K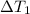 = 159 K - 138 K = 21 K
= 159 K - 138 K = 21 K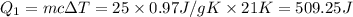
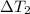 = 223 K - 159 K = 64 K
= 223 K - 159 K = 64 K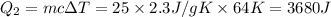
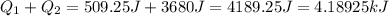 (1kJ=1000J)
(1kJ=1000J)

