
Mrs. smith is demonstrating a chemical change for her class. she places 15 grams of baking soda into a beaker. next she adds 15 grams of vinegar to the same beaker. when the two compounds make contact, they bubble and fizz a great deal. she places the beaker on the balance and notes that the mass of the solution in the beaker is less than the expected 30 grams. why is the mass of the solution in the beaker less than 30 grams? a) the balance was not working correctly. b) the gas that was released changed the mass. c) mass is always lost in a chemical reaction. d) the new products have less mass than the original reactants.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 3


Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
Mrs. smith is demonstrating a chemical change for her class. she places 15 grams of baking soda into...
Questions

Biology, 27.05.2021 01:00



Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00



Mathematics, 27.05.2021 01:00


Chemistry, 27.05.2021 01:00




Mathematics, 27.05.2021 01:00



Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

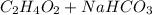 →
→ 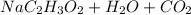 ↑
↑

