
Chemistry, 28.06.2019 22:00 sarbjit879
When potassium metal is placed in water a large amount of energy is released as potassium hydroxide and hydrogen gas are produced in the reaction 2k(s)+2h2o=2koh(aq)+h2(g). your lab partner says this is a redox reaction and a combustion reaction. do you agree? defend your answer by explaining whether or not it meets the requirements of each type of reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
When potassium metal is placed in water a large amount of energy is released as potassium hydroxide...
Questions

English, 18.03.2021 02:30


Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30

Chemistry, 18.03.2021 02:30




Mathematics, 18.03.2021 02:30



Mathematics, 18.03.2021 02:30

Biology, 18.03.2021 02:30







Chemistry, 18.03.2021 02:30


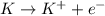 oxidation
oxidation reduction
reduction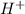 ions of water by gaining electrons (from potassium in water) gives hydrogen gas.
ions of water by gaining electrons (from potassium in water) gives hydrogen gas.


