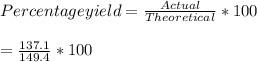Chemistry, 29.06.2019 02:00 101EXPERIENCE
Consider the following reaction: 2h2s (g) + 3o2 (g) 2so2 (g) + 2h2o (g) if o2 was the excess reagent, 8.3 mol of h2s were consumed, and 137.1 g of water were collected after the reaction has gone to completion, what is the percent yield of the reaction? show your work.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
Consider the following reaction: 2h2s (g) + 3o2 (g) 2so2 (g) + 2h2o (g) if o2 was the excess reag...
Questions




Business, 23.07.2020 14:01












Mathematics, 23.07.2020 14:01


History, 23.07.2020 14:01