
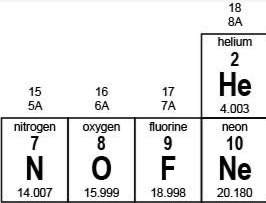
Me! which of the following statements is true? a. neon is more reactive than fluorine because neon needs only one electron to fill its outermost shell. b. neon is more reactive than oxygen because neon has to lose only one electron to fill its outermost shell. c. fluorine is more reactive than nitrogen because fluorine needs only one electron to fill its outermost shell. d. fluorine is more reactive than neon because fluorine has to lose only one electron to fill its outermost shell.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Me! which of the following statements is true? a. neon is more reactive than fluorine because neon...
Questions


Chemistry, 20.11.2019 07:31

Social Studies, 20.11.2019 07:31


Mathematics, 20.11.2019 07:31

History, 20.11.2019 07:31


Mathematics, 20.11.2019 07:31


Mathematics, 20.11.2019 07:31

Mathematics, 20.11.2019 07:31



Mathematics, 20.11.2019 07:31








