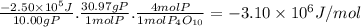Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1


Chemistry, 23.06.2019 13:30
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1
You know the right answer?
When 10.00 g of phosphorus is burned in o2(g) to form p4o10(s), enough heat is generated to raise th...
Questions


English, 05.03.2021 18:40


Mathematics, 05.03.2021 18:40

Mathematics, 05.03.2021 18:40

English, 05.03.2021 18:40


Mathematics, 05.03.2021 18:40



Mathematics, 05.03.2021 18:40



Biology, 05.03.2021 18:40

History, 05.03.2021 18:40


Chemistry, 05.03.2021 18:40


Mathematics, 05.03.2021 18:40

Mathematics, 05.03.2021 18:40