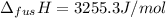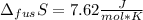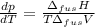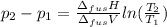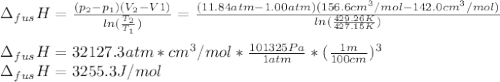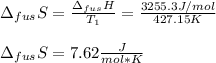The molar volume of a certain solid is 142.0 cm3 mol−1 at 1.00 atm and 427.15 k, its melting temperature. the molar volume of the liquid at this temperature and pressure is 152.6 cm3 mol−1. at 1.2 mpa the melting temperature changes to 429.26 k. calculate the enthalpy and entropy of fusion of the solid

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1


You know the right answer?
The molar volume of a certain solid is 142.0 cm3 mol−1 at 1.00 atm and 427.15 k, its melting tempera...
Questions

English, 28.10.2019 21:31

Health, 28.10.2019 21:31


Mathematics, 28.10.2019 21:31

Social Studies, 28.10.2019 21:31

English, 28.10.2019 21:31

Mathematics, 28.10.2019 21:31

Mathematics, 28.10.2019 21:31

History, 28.10.2019 21:31

Physics, 28.10.2019 21:31

Mathematics, 28.10.2019 21:31

Social Studies, 28.10.2019 21:31


Biology, 28.10.2019 21:31

Mathematics, 28.10.2019 21:31

Social Studies, 28.10.2019 21:31

Mathematics, 28.10.2019 21:31

Mathematics, 28.10.2019 21:31

Mathematics, 28.10.2019 21:31