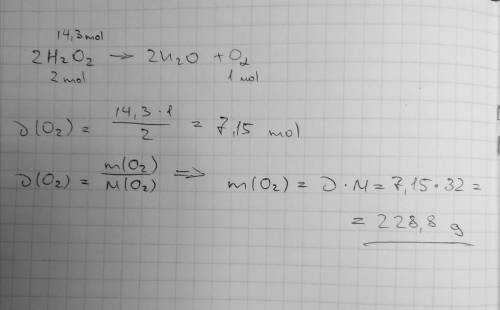Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Mm h2o2 = 34.02 g/mol mm h2o = 18.02 g/mol mm o2 = 32 g/mol 2h2o2 —> 2h2o + o2 if 14.3 moles of...
Questions



Mathematics, 15.01.2021 19:20

Social Studies, 15.01.2021 19:20

Mathematics, 15.01.2021 19:20

Mathematics, 15.01.2021 19:20

Mathematics, 15.01.2021 19:20

Mathematics, 15.01.2021 19:20




Mathematics, 15.01.2021 19:20

English, 15.01.2021 19:20

Computers and Technology, 15.01.2021 19:20

English, 15.01.2021 19:20


Mathematics, 15.01.2021 19:20

Chemistry, 15.01.2021 19:20

Mathematics, 15.01.2021 19:20

Arts, 15.01.2021 19:20