
Chemistry, 29.06.2019 08:30 Calumworthy6046
Asealed container was filled with 0.300 mol h2(g), 0.400 mol i2 (g), and 0.200 mol hi (g) at 870k and total pressure 1.00 bar. calculate the amounts of the components in the mixture at equilibrium given that k = 870 for the reaction h2 (g) + i2 (g) ⇜ 2 hi (g).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
Asealed container was filled with 0.300 mol h2(g), 0.400 mol i2 (g), and 0.200 mol hi (g) at 870k an...
Questions

English, 26.01.2021 20:00


Arts, 26.01.2021 20:00

Mathematics, 26.01.2021 20:00

Chemistry, 26.01.2021 20:00



History, 26.01.2021 20:00



English, 26.01.2021 20:00

History, 26.01.2021 20:00

Mathematics, 26.01.2021 20:00

Physics, 26.01.2021 20:00



English, 26.01.2021 20:00

History, 26.01.2021 20:00

Mathematics, 26.01.2021 20:00

Mathematics, 26.01.2021 20:00

![[HI] _{eq}=0.825mol](/tpl/images/0030/3484/a93b2.png)
![[H_2] _{eq}=0.010mol](/tpl/images/0030/3484/29662.png)
![[I_2] _{eq}=0.078mol](/tpl/images/0030/3484/3a542.png)
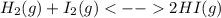
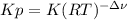 ):
):![\frac{Kp}{(RT)^{2-2} }=\frac{[HI]^{2}_{eq} }{[H_2]_{eq}[I_2]_{eq}} \\Kp=\frac{[HI]^{2}_{eq} }{[H_2]_{eq}[I_2]_{eq}}](/tpl/images/0030/3484/98372.png)
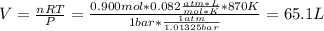
![[H_2] _0=\frac{0.300mol}{65.1L}=0.005M](/tpl/images/0030/3484/0ef57.png)
![[I_2] _0=\frac{0.400mol}{65.1L}=0.006M](/tpl/images/0030/3484/d9a8e.png)
![[HI] _0=\frac{0.200mol}{65.1L}=0.003M](/tpl/images/0030/3484/e3fd2.png)
 due to the equilibrium:
due to the equilibrium: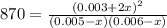
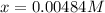
![[HI] _{eq} =(0.003M +2(0.00484M))*65.1L=0.825mol](/tpl/images/0030/3484/1d681.png)
![[H_2] _{eq} =(0.005M -0.00484M)*65.1L=0.010mol](/tpl/images/0030/3484/34688.png)
![[I_2] _{eq} =(0.006M -0.00484M)*65.1L=0.078mol](/tpl/images/0030/3484/28b57.png)


