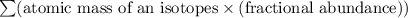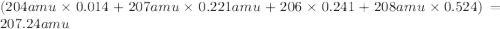Chemistry, 29.06.2019 14:30 espiritu7470
There are four isotopes of lead and their relative abundance listed below. use this information to calculate the approximate atomic mass of lead. show all work (4 points) 82 protons, 122 neutrons, 1.4% 82 protons, 125 neutrons, 22.1% 82 protons, 124 neutrons, 24.1% 82 protons, 126 neutrons, 52.4% show !

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
There are four isotopes of lead and their relative abundance listed below. use this informati...
Questions




Mathematics, 15.07.2020 02:01




Chemistry, 15.07.2020 02:01



Mathematics, 15.07.2020 02:01



Mathematics, 15.07.2020 02:01



Mathematics, 15.07.2020 02:01