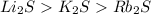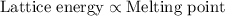Chemistry, 29.06.2019 16:30 dukkchild666
The melting points for the compounds li2s, rb2s, and k2s are 900°c, 530°c, and 840°c, respectively. list these three compounds in order of increasing lattice energy.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
The melting points for the compounds li2s, rb2s, and k2s are 900°c, 530°c, and 840°c, respectively....
Questions


Mathematics, 01.12.2019 04:31

Mathematics, 01.12.2019 04:31


Social Studies, 01.12.2019 04:31


Mathematics, 01.12.2019 04:31

Mathematics, 01.12.2019 04:31

Mathematics, 01.12.2019 04:31


Computers and Technology, 01.12.2019 04:31

Mathematics, 01.12.2019 04:31

Mathematics, 01.12.2019 04:31

Computers and Technology, 01.12.2019 04:31

Mathematics, 01.12.2019 04:31





History, 01.12.2019 04:31