
Chemistry, 29.06.2019 22:30 mayamcmillan11
This chemical equation is not balanced: agbr(s) → ag(s) + br2(g). why isn’t it possible for the reaction to occur as indicated by the unbalanced equation? a. there are more substances on the right side of the equation than on the left side. b. there is a gas on the right side of the equation but not on the left side. c. the bromine atoms must go through a liquid state before becoming a gas. d. there is more mass represented on the right side of the equation than on the left side. e. the bromine atoms on the right side of the equation are not bonded to another element.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
This chemical equation is not balanced: agbr(s) → ag(s) + br2(g). why isn’t it possible for the rea...
Questions



English, 17.05.2021 18:40


Mathematics, 17.05.2021 18:40

History, 17.05.2021 18:40


Mathematics, 17.05.2021 18:40

Mathematics, 17.05.2021 18:40

Mathematics, 17.05.2021 18:40

Mathematics, 17.05.2021 18:40


Social Studies, 17.05.2021 18:40

English, 17.05.2021 18:40



Mathematics, 17.05.2021 18:40


Biology, 17.05.2021 18:40

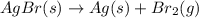 (unbalanced reaction)
(unbalanced reaction)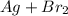 = 108 + 2(80) = 268 g
= 108 + 2(80) = 268 g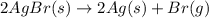 (Balanced reaction)
(Balanced reaction)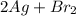 = 2(108) + 2(80) = 376 g
= 2(108) + 2(80) = 376 g

