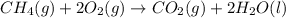Chemistry, 30.06.2019 00:00 alexwlodko
The combustion of methane gas (ch4) forms co2(g)+ h2o(ℓ). calculate the heat produced by burning 1.9 mol of the methane gas. the standard molar enthalpy of combustion of methane is 890.2kj/mol. answer in units of kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2


Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
You know the right answer?
The combustion of methane gas (ch4) forms co2(g)+ h2o(ℓ). calculate the heat produced by burning 1.9...
Questions

Mathematics, 05.02.2021 01:00

Engineering, 05.02.2021 01:00


Computers and Technology, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00


Biology, 05.02.2021 01:00


Social Studies, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00



English, 05.02.2021 01:00


Chemistry, 05.02.2021 01:00

English, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00