Chemistry, 30.06.2019 00:30 shels10tay
An unbalanced chemical equation is shown: 2cao + 2h2o → ca(oh)2 which of the following statements explains why the equation is not balanced? four molecules of ca(oh)2 should be produced during the decomposition reaction. two molecules of ca(oh)2 should be produced during the decomposition reaction. four molecules of ca(oh)2 should be produced during the synthesis reaction. two molecules of ca(oh)2 should be produced during the synthesis reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1



Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
An unbalanced chemical equation is shown: 2cao + 2h2o → ca(oh)2 which of the following statements e...
Questions















Business, 30.08.2020 14:01





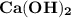 should be produced during the synthesis reaction.
should be produced during the synthesis reaction.