
In chemical formulas, there are often subscript numbers (such as the 2 in h2o, or the 6, 12, and 6 in c6h12o6). what do these subscript numbers mean? what information do the tell us? question options: subscript numbers tell us how many of the element to their upper-left there are in the molecule. subscript numbers tell us the electrical charge of the element to their upper-left. subscript numbers tell us how many of the element to their upper-left there are. subscript numbers tell us the electrical charge of the element to their upper-right. subscript numbers tell us how many of the element to their upper-right there are in the molecule. subscript numbers tell us how many of the element to their upper-right there are.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3



Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
You know the right answer?
In chemical formulas, there are often subscript numbers (such as the 2 in h2o, or the 6, 12, and 6 i...
Questions


Business, 29.09.2021 16:40



Mathematics, 29.09.2021 16:40

Mathematics, 29.09.2021 16:40


Mathematics, 29.09.2021 16:40


Social Studies, 29.09.2021 16:50

Biology, 29.09.2021 16:50


Mathematics, 29.09.2021 16:50




Mathematics, 29.09.2021 16:50


English, 29.09.2021 16:50

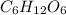 is the chemical formula of the glucose molecule. The glucose molecule is made up of six carbon atoms, twelve hydrogen atoms and 6 oxygen atoms.
is the chemical formula of the glucose molecule. The glucose molecule is made up of six carbon atoms, twelve hydrogen atoms and 6 oxygen atoms. 

