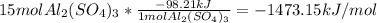Chemistry, 30.06.2019 19:00 lilchannelll4125
Al2(so4)3(s) + h2o(l) al2o3(s) + h2so4 (aq) calculate enthalpy formation for this reaction. balance the reaction. calculate the total enthalpy change that would occur from 15 moles of al2(so4)3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
Al2(so4)3(s) + h2o(l) al2o3(s) + h2so4 (aq) calculate enthalpy formation for this reaction. balance...
Questions

History, 21.08.2020 14:01

Mathematics, 21.08.2020 14:01



Spanish, 21.08.2020 14:01


English, 21.08.2020 14:01

Computers and Technology, 21.08.2020 14:01

Mathematics, 21.08.2020 14:01

Mathematics, 21.08.2020 14:01


Computers and Technology, 21.08.2020 14:01


English, 21.08.2020 14:01

English, 21.08.2020 14:01



English, 21.08.2020 14:01

Mathematics, 21.08.2020 14:01



![H_{reaction}^{0}=[H_{f}^{0}(Al_{2}O_{3}(s)) + (3*H_{f}^{0}(H_{2}SO_{4}(aq))] - [H_{f}^{0}(Al_{2}SO_{4}(aq)) + (3*H_{f}^{0}(H_{2}O(l))]](/tpl/images/0035/8926/80689.png)
 reacts will be=
reacts will be=