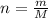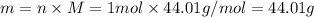Chemistry, 30.06.2019 19:00 beastboy13
The molar mass of co2 is 44.01 g/mole. choose which of the following correctly represents this relationship correctly as a conversion factor. 1 mol co2 = 44.01 g co2 44.01 mol co2 = 1 g co2 44.01 molecules co2 = 1 g co2 1 molecule co2 = 44.01 g co2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1


Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
The molar mass of co2 is 44.01 g/mole. choose which of the following correctly represents this relat...
Questions

Physics, 16.12.2020 14:00

English, 16.12.2020 14:00



Mathematics, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00

History, 16.12.2020 14:00

Chemistry, 16.12.2020 14:00



Mathematics, 16.12.2020 14:00

Physics, 16.12.2020 14:00



Health, 16.12.2020 14:00

Physics, 16.12.2020 14:00



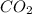 = 44.01 grams of
= 44.01 grams of