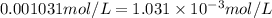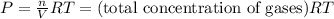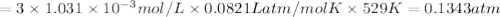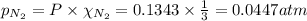Chemistry, 30.06.2019 21:30 nathaliapachon1254
For the reaction n2(g) + 3 h2(g) = 2 nh3(g), kp = 0.0489 at 256˚c. for parts a through d, assume that the reaction is at this temperature. c) a third equilibrium mixture contains 0.0100 mol/l of n2 and 0.0300 mol/l of nh3.what is the concentration of h2 in this mixture? d) a fourth equilibrium mixture contains equal concentrations of all three chemicals. what is the pressure of each substance in this mixture? me on c & d by 11/25 11pm !


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

You know the right answer?
For the reaction n2(g) + 3 h2(g) = 2 nh3(g), kp = 0.0489 at 256˚c. for parts a through d, assume tha...
Questions



Mathematics, 18.07.2019 12:30






History, 18.07.2019 12:30

Spanish, 18.07.2019 12:30

Geography, 18.07.2019 12:30


Mathematics, 18.07.2019 12:30

Mathematics, 18.07.2019 12:30


Chemistry, 18.07.2019 12:30


Mathematics, 18.07.2019 12:30


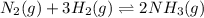
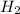 in this mixture
in this mixture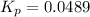
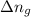 :
: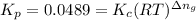
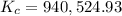
![K_c=940,524.93=\frac{[NH_3]^2}{[N_2][H_2]^3}=\frac{(0.0300 mol/L)^3}{0.0100 mol/L\times [H_2]^3}](/tpl/images/0036/3201/0fc67.png)
![[H_2]^3=10,450,277](/tpl/images/0036/3201/9b237.png)
![[H_2]=218.62 mol/L](/tpl/images/0036/3201/d8502.png)
![[N_2]=[H_2]=[NH_3]=x mol/L](/tpl/images/0036/3201/a39fc.png)
![K_c=940,524.93=\frac{[NH_3]^2}{[N_2][H_2]^3}=\frac{x^2}{x\times x^3}=\frac{1}{x^2}](/tpl/images/0036/3201/ddb76.png)