
Chemistry, 30.06.2019 23:30 sssavannahh
21. list 3 indicators of a chemical change. a. b. c. 22. check the following problem for errors. if you find an error, identify it, tell why it is an error and correct the error solving the problem correctly. convert 0.45 g zinc hydroxide to moles. (hint: there are errors) 0.65 g znoh 82.41 mol = 3.22 x 10 25 mol 6.02x1023 g 23. what is an ion? explain how ions form. distinguish between a cation and an anion. be sure to use at least 3 to 4 complete content related sentences in your explanation. 24. write the symbol for the ion formed when each element gains electrons and attains a noble-gas electron configuration. a. br b. h c. se 25. name each of the compounds. there are ionic, covalent and acid/base compounds in the list. a. n2h4 b. pi3 c. lioh

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1


Chemistry, 23.06.2019 14:50
Use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 +473 kj +206 kj -392 kj -91 kj -486 kj
Answers: 1
You know the right answer?
21. list 3 indicators of a chemical change. a. b. c. 22. check the following problem for errors....
Questions

Mathematics, 23.04.2020 06:46

English, 23.04.2020 06:46

Mathematics, 23.04.2020 06:46

Mathematics, 23.04.2020 06:46


Mathematics, 23.04.2020 06:46


Mathematics, 23.04.2020 06:46

Mathematics, 23.04.2020 06:46

English, 23.04.2020 06:46

Mathematics, 23.04.2020 06:46


Mathematics, 23.04.2020 06:46


Chemistry, 23.04.2020 06:46

Physics, 23.04.2020 06:46


Mathematics, 23.04.2020 06:47


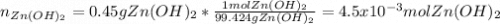
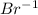 as it has seven valance electrons, it needs one electron to attain the octet.
as it has seven valance electrons, it needs one electron to attain the octet.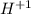 as it loses one electron when forming a cation.
as it loses one electron when forming a cation.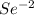 as it has six valance electrons, it needs two electrons to attain the octet.
as it has six valance electrons, it needs two electrons to attain the octet.


