
Chemistry, 01.07.2019 00:00 kianofou853
Kw, the equilibrium constant for the ionization of water by the equation below, is 1.0 x 10-14. what does that mean when we are considering pure water?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1


Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1
You know the right answer?
Kw, the equilibrium constant for the ionization of water by the equation below, is 1.0 x 10-14. what...
Questions



Spanish, 07.06.2020 08:58






Mathematics, 07.06.2020 08:58


Social Studies, 07.06.2020 08:58

English, 07.06.2020 08:58

Mathematics, 07.06.2020 08:58


Biology, 07.06.2020 08:58




Mathematics, 07.06.2020 08:58

![[H^+]\&[OH^-]](/tpl/images/0036/7175/bc884.png) are equal in pure water.
are equal in pure water.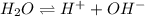
![K_{eq}=\frac{[H^+][OH^-]}{[H_2O]}](/tpl/images/0036/7175/59636.png)
![K_w=K_{eq}[H_2O]=[H^+][OH^-]=1.0\times 10^{-14}](/tpl/images/0036/7175/f0cd4.png)
![pH=7=-\log[H^+]](/tpl/images/0036/7175/ae186.png)
![[H^+]=1\times 10^{-7}mol/L](/tpl/images/0036/7175/f6d22.png)
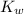 of pure water is given
of pure water is given![K_w=[H^+][OH^-]=1\times 10^{-7}\times[OH^-]= 1\times 10^{-14}](/tpl/images/0036/7175/db7b7.png)
![[OH^-]=1\times 10^{-7}mol/L](/tpl/images/0036/7175/b6b71.png)
![[H^+]\&[OH^-]](/tpl/images/0036/7175/72025.png) are equal in pure water
are equal in pure water

