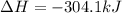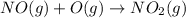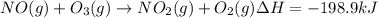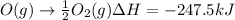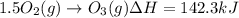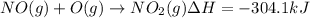Chemistry, 01.07.2019 00:30 jerseygirl1783
Calculate the enthalpy change for the reaction no(g) + o(g) → no2(g) from the following data: no(g) + o3(g) → no2(g) + o2(g) δh = –198.9 kj o3(g) → 1.5o2(g) δh = –142.3 kj o2(g) → 2o(g) δh = 495.0 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
You know the right answer?
Calculate the enthalpy change for the reaction no(g) + o(g) → no2(g) from the following data: no(g)...
Questions

Mathematics, 30.10.2020 01:00

Chemistry, 30.10.2020 01:00






Biology, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00


Biology, 30.10.2020 01:00




Mathematics, 30.10.2020 01:00




Mathematics, 30.10.2020 01:00

Biology, 30.10.2020 01:00