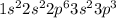Chemistry, 01.07.2019 00:30 baeethtsadia
(5 points) 2. a neutral atom of phosphorus has 15 electrons. explain why the electron configuration below is not the correct configuration for a neutral atom of phosphorus in its ground state. 1s2 2s2 2p6 3s2 3p2 4s1 (5 points) 3. find rubidium, magnesium, and aluminum on the periodic table. fill in the table below based on the locations of these metals on the periodic table. be thorough in filling in the far right column! element symbol group number number of valence electrons general reactivity of the metal with an explanation for this reactivity based on the number of valence electrons rubidium magnesium aluminum

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1


You know the right answer?
(5 points) 2. a neutral atom of phosphorus has 15 electrons. explain why the electron configuration...
Questions

Biology, 23.04.2020 03:35

Business, 23.04.2020 03:35



English, 23.04.2020 03:35

Mathematics, 23.04.2020 03:35

Social Studies, 23.04.2020 03:35


Mathematics, 23.04.2020 03:35


Mathematics, 23.04.2020 03:35


Mathematics, 23.04.2020 03:35

Mathematics, 23.04.2020 03:35

Mathematics, 23.04.2020 03:35





Chemistry, 23.04.2020 03:35