
Chemistry, 01.07.2019 01:30 iiwolfiexuni
The hydrogen atom is not actually electronegative enough to form bonds to xenon. were the xenon-hydrogen bond to exist, what would be the structure of xeh4? double-click any atom and type xe to change the label. draw the molecule by placing atoms on the grid and connecting them with bonds. show all lone pairs of electrons.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2


Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1
You know the right answer?
The hydrogen atom is not actually electronegative enough to form bonds to xenon. were the xenon-hydr...
Questions




History, 24.03.2020 00:29

Mathematics, 24.03.2020 00:29


Mathematics, 24.03.2020 00:29


English, 24.03.2020 00:29



Mathematics, 24.03.2020 00:29

Chemistry, 24.03.2020 00:29







Biology, 24.03.2020 00:29

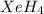 will be square-planar.
will be square-planar.

