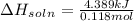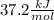Chemistry, 01.07.2019 03:00 paigeisawesome
Student conducts an experiment to determine the enthalpy of solution for lithium chloride dissolved in water. the student combines 5.00 grams of lithium chloride with 100.0 ml of distilled water. the initial temperature of the water is 23.0â°c and the highest temperature after mixing reaches 33.0â°c. assume a density of 1.00 g/ml and a specific heat of 4.18 .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
Student conducts an experiment to determine the enthalpy of solution for lithium chloride dissolved...
Questions


Mathematics, 16.11.2019 02:31


Computers and Technology, 16.11.2019 02:31

Mathematics, 16.11.2019 02:31

Mathematics, 16.11.2019 02:31


Mathematics, 16.11.2019 02:31


Biology, 16.11.2019 02:31







Biology, 16.11.2019 02:31



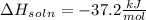
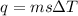
 = change in temperature
= change in temperature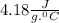
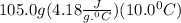
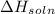 we convert q to kJ and divide by the moles of solute.
we convert q to kJ and divide by the moles of solute.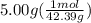
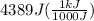 = 4.389 kJ
= 4.389 kJ