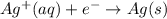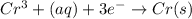Chemistry, 01.07.2019 06:30 trosclairozlynn02
The following equations are half reactions and reduction potentials. ag+ (aq) + e– ag(s) has a reduction potential of +0.80 v. cr3+ (aq) + 3e– cr(s) has a reduction potential of –0.74 v. which statement best compares the substances in these half reactions? a silver ion gains electrons more easily and is a stronger oxidizing agent than a chromium(iii) ion. a silver ion loses electrons more easily and is a stronger oxidizing agent than a chromium(iii) ion. a silver ion gains electrons more easily and is a stronger reducing agent than a chromium(iii) ion. a silver ion loses electrons more easily and is a stronger reducing agent than a chromium(iii) ion. the answer is a

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1


Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
The following equations are half reactions and reduction potentials. ag+ (aq) + e– ag(s) has a reduc...
Questions

Mathematics, 19.05.2021 06:30

Arts, 19.05.2021 06:30

Spanish, 19.05.2021 06:30

English, 19.05.2021 06:30




English, 19.05.2021 06:30


Mathematics, 19.05.2021 06:30

Mathematics, 19.05.2021 06:30


Mathematics, 19.05.2021 06:30


Spanish, 19.05.2021 06:30


Chemistry, 19.05.2021 06:30

Mathematics, 19.05.2021 06:30