
Chemistry, 01.07.2019 07:00 randyg0531
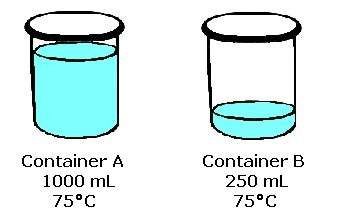
Look at the two containers of water and their starting conditions shown above. how will the temperatures of the water in the containers compare if an equal amount of heat is absorbed by both containers of water without boiling? a. both water temperatures will increase by the same amount. b. both water temperatures will increase, but container a's will increase more. c. both water temperatures will decrease, but container b's will decrease more. d. both water temperatures will increase, but container b's will increase more.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
Look at the two containers of water and their starting conditions shown above. how will the temperat...
Questions



Mathematics, 14.08.2020 16:01





English, 14.08.2020 16:01







Mathematics, 14.08.2020 16:01



Physics, 14.08.2020 16:01




