
Chemistry, 01.07.2019 08:30 andrea1704
The formation of cscl from cs(s) and cl2 (g) involves the following steps: cs(s) -> cs(g) 1/2cl2(g) -> cl(g) cs(g) -> cs + (g) + e – cl(g) + e - -> cl - (g) cs+(g) + cl - (g) -> cscl(s) which of these steps absorb energy and which release energy?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
The formation of cscl from cs(s) and cl2 (g) involves the following steps: cs(s) -> cs(g) 1/2...
Questions

Mathematics, 06.04.2021 02:50


History, 06.04.2021 02:50

History, 06.04.2021 02:50

Mathematics, 06.04.2021 02:50


English, 06.04.2021 02:50


History, 06.04.2021 02:50


English, 06.04.2021 02:50

Physics, 06.04.2021 02:50

English, 06.04.2021 02:50


Social Studies, 06.04.2021 02:50


Computers and Technology, 06.04.2021 02:50


Mathematics, 06.04.2021 02:50

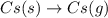
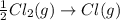
 gas in order to break bond between two Cl atoms to form isolated(alone) single chlorine atom.So, the energy will be absorbed by the chlorine gas molecule in this reaction.
gas in order to break bond between two Cl atoms to form isolated(alone) single chlorine atom.So, the energy will be absorbed by the chlorine gas molecule in this reaction.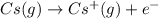
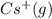 cation. In order to remove an electron from the outer most shell of Cs atom energy will be required by Cs atom, So, energy will be absorbed in this reaction.
cation. In order to remove an electron from the outer most shell of Cs atom energy will be required by Cs atom, So, energy will be absorbed in this reaction.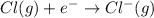
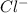 anion from Cl atom in gaseous state. Chlorine atom need one electron to attain noble gas configuration. So, when an electron is added to the outer most shell of chlorine it attains stability of fully filled outermost shell by which it releases energy on addition of an electron.
anion from Cl atom in gaseous state. Chlorine atom need one electron to attain noble gas configuration. So, when an electron is added to the outer most shell of chlorine it attains stability of fully filled outermost shell by which it releases energy on addition of an electron.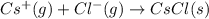
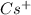 cation and
cation and 

