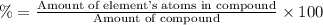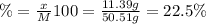Chemistry, 01.07.2019 11:30 cmflores3245
A50.51g sample of a compound made from phosphorus and chlorine is decomposed. analysis of the products showed that 11.39 g of phosphorus atoms were produced. answer using three significant figures. what is the percent by mass of phosphorus? % what is the percent by mass of chlorine? %

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
A50.51g sample of a compound made from phosphorus and chlorine is decomposed. analysis of the produc...
Questions

Health, 11.07.2019 20:10


English, 11.07.2019 20:10

Biology, 11.07.2019 20:10


Business, 11.07.2019 20:10

Business, 11.07.2019 20:10

Biology, 11.07.2019 20:10



Mathematics, 11.07.2019 20:10

Mathematics, 11.07.2019 20:10


Chemistry, 11.07.2019 20:10

History, 11.07.2019 20:10

Geography, 11.07.2019 20:10



Mathematics, 11.07.2019 20:10

English, 11.07.2019 20:10