
Chemistry, 01.07.2019 14:00 jc95704816
How many grams of cah2 are needed to generate 48.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1


Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
How many grams of cah2 are needed to generate 48.0 l of h2 gas at a pressure of 0.995 atm and a temp...
Questions

Advanced Placement (AP), 24.01.2022 23:40


Engineering, 24.01.2022 23:40

Mathematics, 24.01.2022 23:40


Mathematics, 24.01.2022 23:40



English, 24.01.2022 23:40






Mathematics, 24.01.2022 23:40


Mathematics, 24.01.2022 23:40

Mathematics, 24.01.2022 23:40

Mathematics, 24.01.2022 23:40

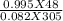 = 1.90 moles [using PV=nRT equation where, n=
= 1.90 moles [using PV=nRT equation where, n= 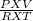 ]
]

