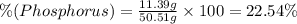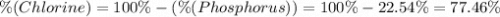Chemistry, 01.07.2019 17:00 hobbs4ever1
A50.51g sample of a compound made from phosphorus and chlorine is decomposed. analysis of the products showed that 11.39 g of phosphorus atoms were produced. answer using three significant figures. what is the percent by mass of phosphorus? % what is the percent by mass of chlorine? %

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

You know the right answer?
A50.51g sample of a compound made from phosphorus and chlorine is decomposed. analysis of the produc...
Questions




History, 07.04.2020 20:11

English, 07.04.2020 20:12

English, 07.04.2020 20:12

English, 07.04.2020 20:12






History, 07.04.2020 20:12

Mathematics, 07.04.2020 20:12

Computers and Technology, 07.04.2020 20:12


History, 07.04.2020 20:12