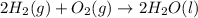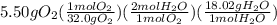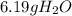Chemistry, 02.07.2019 01:00 natalie2sheffield
Consider the reaction 2h2(g)+o2(g)→2h2o(l) what is the mass of water, h2o(l), produced when 5.50 g of o2(g) reacts with excess h2(g)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Consider the reaction 2h2(g)+o2(g)→2h2o(l) what is the mass of water, h2o(l), produced when 5.50 g o...
Questions

Arts, 23.07.2019 01:30




Mathematics, 23.07.2019 01:30


History, 23.07.2019 01:30

Social Studies, 23.07.2019 01:30



Physics, 23.07.2019 01:30

Mathematics, 23.07.2019 01:30




History, 23.07.2019 01:30

Biology, 23.07.2019 01:30


Mathematics, 23.07.2019 01:30

Geography, 23.07.2019 01:30