
You discover a chemical reaction that produces 2 moles of valuable propane for every 8 moles of carbon supplied. essentially, this is an exchange rate of 2 moles of propane for every 8 moles of carbon. if you have 1.7 moles of carbon, how many moles of propane can you produce using this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
You discover a chemical reaction that produces 2 moles of valuable propane for every 8 moles of carb...
Questions



English, 12.02.2021 19:00

Mathematics, 12.02.2021 19:00


Mathematics, 12.02.2021 19:00

Mathematics, 12.02.2021 19:00



English, 12.02.2021 19:00

Mathematics, 12.02.2021 19:00

Mathematics, 12.02.2021 19:00



Physics, 12.02.2021 19:00


Mathematics, 12.02.2021 19:00



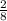 ×1.7 = 0.425 moles
×1.7 = 0.425 moles

