
Chemistry, 02.07.2019 01:00 pattydixon6
An unbalanced chemical equation is shown: h2o2 → 2h2o + o2 which of the following statements explains why the equation is not balanced? a two h2o2 molecules should decompose to form the given products. b four h2o2 molecules should decompose to form the given products. c two h2o2 molecules should undergo a synthesis reaction to form the given products. d four h2o2 molecules should undergo a synthesis reaction to form the given products.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1



Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
An unbalanced chemical equation is shown: h2o2 → 2h2o + o2 which of the following statements explai...
Questions

Mathematics, 24.07.2019 19:30

History, 24.07.2019 19:30




Mathematics, 24.07.2019 19:30

History, 24.07.2019 19:30

Social Studies, 24.07.2019 19:30

Computers and Technology, 24.07.2019 19:30

Social Studies, 24.07.2019 19:30

Physics, 24.07.2019 19:30


Mathematics, 24.07.2019 19:30

Social Studies, 24.07.2019 19:30

History, 24.07.2019 19:30

History, 24.07.2019 19:30

Mathematics, 24.07.2019 19:30



History, 24.07.2019 19:30

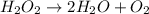
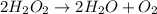
 molecules should decompose to form the given products."
molecules should decompose to form the given products."

