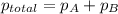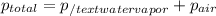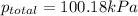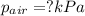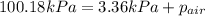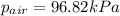Chemistry, 02.07.2019 02:30 onlymyworld27
You collect a sample of gases from an indoor pool area. the sample contains air and water vapor. the total pressure is 100.18 kilopascals, and the partial pressure of the water vapor is 3.36 kilopascals. what is the partial pressure of the air in the sample? a. 29.8 kpa b. 51.77 kpa c. 96.82 kpa d. 103.54 kpa e. 337 kpa

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
You collect a sample of gases from an indoor pool area. the sample contains air and water vapor. the...
Questions

Computers and Technology, 14.07.2019 05:10



Mathematics, 14.07.2019 05:10

Mathematics, 14.07.2019 05:10


Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Computers and Technology, 14.07.2019 05:10

Mathematics, 14.07.2019 05:10