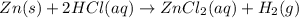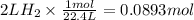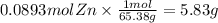How many grams of zinc metal are required to produce 2.00 liters of hydrogen gas at stp according to the chemical equation shown below? how many grams of zinc metal are required to produce 2.00 liters of hydrogen gas at stp according to the chemical equation shown below? 5.83 g 11.7 g 0.171 g 131 g?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
How many grams of zinc metal are required to produce 2.00 liters of hydrogen gas at stp according to...
Questions





Mathematics, 10.09.2019 07:20


Mathematics, 10.09.2019 07:20



English, 10.09.2019 07:20

English, 10.09.2019 07:20


Mathematics, 10.09.2019 07:20



English, 10.09.2019 07:20

Mathematics, 10.09.2019 07:20

Mathematics, 10.09.2019 07:20

Mathematics, 10.09.2019 07:20