
Chemistry, 02.07.2019 04:00 qveenjordan6456
Which options correctly describe similarities between the elements boron (b), aluminum (al), and gallium (ga)? select all that apply. hint: review your periodic table. all three elements are part of the boron family. all three elements tend to form ionic compounds that cannot be dissolved in water. all three elements tend to form 3+ cations when reacting with metals. all three elements have five valence electrons.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
Which options correctly describe similarities between the elements boron (b), aluminum (al), and gal...
Questions




English, 10.07.2019 08:00



Social Studies, 10.07.2019 08:00

Mathematics, 10.07.2019 08:00


History, 10.07.2019 08:00

Chemistry, 10.07.2019 08:00








Spanish, 10.07.2019 08:00

English, 10.07.2019 08:00

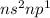 .Total number of valence electron in each element is 3.These elements show (+3) oxidation state. So, these elements tend to form (+3) cations when reacting with metals.
.Total number of valence electron in each element is 3.These elements show (+3) oxidation state. So, these elements tend to form (+3) cations when reacting with metals.


