
Which of the following statements is true about the strength of the intermolecular forces in ch4 and nh3? a: ch4 ≥ nh3 because ch4 is tetrahedral but nh3 is pyramidal. b: ch4 < nh3 because δ− on c in the ch bond is greater than δ− on n in the nh bond. c: ch4 < nh3 because the nh bond is more polar than the ch bond. d: ch4 ≥ nh3 because ch4 has h bonding but nh3 has dispersion forces.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

You know the right answer?
Which of the following statements is true about the strength of the intermolecular forces in ch4 and...
Questions

Computers and Technology, 21.03.2020 03:23





Biology, 21.03.2020 03:24



Mathematics, 21.03.2020 03:24


History, 21.03.2020 03:24


Mathematics, 21.03.2020 03:24



Mathematics, 21.03.2020 03:25



Physics, 21.03.2020 03:25

Mathematics, 21.03.2020 03:26

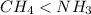 because the NH bond is more polar than CH bond.
because the NH bond is more polar than CH bond.

